The following abstract is written by: Isabelle Guinobert, Claude Blondeau, Bruno Colicchio, Noufissa Oudrhiri, Alain Dieterlen, Eric Jeandidier, Georges Deschenes, Valérie Bardot, César Cotte,
Isabelle Ripoche, Patrice Carde, Lucile Berthomier and Radhia M’Kacher.
Submission received: 19 December 2019 / Revised: 6 February 2020 / Accepted: 7 February 2020 / Published: 12 February 2020 (This article belongs to the Special Issue
Oxidative Stress and Inflammation: From Mechanisms to Therapeutic Approaches)
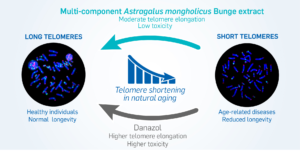
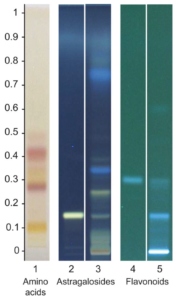
A link between telomere shortening and oxidative stress was found in aging people and patients with cancer or inflammatory diseases. Extracts of Astragalus spp. are known to stimulate telomerase activity, thereby compensating telomere shortening. We characterized a multi-component hydroethanolic root extract (HRE) of Astragalus mongholicus Bunge and assessed its effects on telomeres compared to those of danazol. Astragalosides I to IV, flavonoids, amino acids and sugars were detected in the HRE. Samples of peripheral blood lymphocytes with short telomeres from 18 healthy donors (mean age 63.5 years; range 3286 years) were exposed to a single dose of 1 µg/mL HRE or danazol for three days. Telomere length and telomerase expression were then measured. Significant elongation of telomeres associated to a less toxicity was observed in lymphocytes from 13/18 donors following HRE treatment (0.54 kb (0.15-2.06 kb)) and in those from 9/18 donors after danazol treatment (0.95 kb (0.06-2.06 kb)). The rate of cells with short telomeres (<3 kb) decreased in lymphocytes from all donors after exposure to either HRE or danazol, telomere elongation being telomerase-dependent. These findings suggest that the HRE could be used for the management of age-related diseases.
Telomeres are dynamic nucleoprotein structures that protect the ends of chromosomes from degradation and activation of the DNA damage response. Telomeres are considered to be a biological clock, playing a major role in aging and genome stability [
7]. It is now well-documented that telomere dysfunction is a potential biomarker for age-related diseases and can contribute to the prognosis of several diseases [
8,
9,
10,
11,
12]. Telomere sensitivity to inflammation and oxidative stress such as ionizing radiation has been previously demonstrated [
13]. This sensitivity promotes telomere shortening and replicative senescence [
14] leading to chromosomal instability [
15]. This phenomenon was found to occur in both proliferative and nonproliferative tissues [
16]. To overcome telomere dysfunction, activation of a telomere maintenance mechanism is required to support cell proliferation and immortalization. In most cases, telomeres are elongated by telomerase, a cellular reverse transcriptase capable of compensating telomere shortening through de novo addition of (T2AG3)n [
17]. However, telomerase activity can decline with age [
18]. Telomerase reactivation approaches have been investigated to counteract telomere shortening and its consequences and have consequently been proposed for the treatment of age-related diseases and telomeropathies [
19]. It was shown that antioxidant and anti-inflammatory agents can be used to slow the loss of telomere length [
20]. Furthermore, several studies have reported telomere elongation by androgens such as danazol [
7,
21] and by herbal products [
22,
23,
24,
25,
26,
27].
In traditional Chinese medicine,
Astragali Radix (known as Huang Qi), the dried root of
Astragalus membranaceus (Fisch.) Bunge or
Astragalus mongholicus Bunge, is an herbal medicine that has been used to counteract oxidative stress, inflammation and aging since ancient times [
28,
29]. Pharmacological studies have shown that
Astragalus spp. extracts and their principal components (saponins, flavonoids and polysaccharides) act on aging via several mechanisms [
23,
30]. In addition to their antioxidant, anti-inflammatory, immunoregulatory and anticancer effects, extracts of
Astragalus spp. have been shown to exert beneficial effects on telomeres and to stimulate telomerase activity in various models [
22,
23,
31]. Most studies investigated cycloastragenol (TA-65), a single chemical entity isolated by a proprietary purification process from a root extract of
Astragalus membranaceus [
22,
24,
25,
31,
32].
The objective of this study was to determine the phytochemical composition of a multicomponent hydroethanolic root extract (HRE) of Astragalus mongholicus Bunge and to assess its effects on telomere length and telomerase expression in lymphocytes from healthy donors with short telomeres, per comparison with those of danazol, no such comparison having been made up to now within the same cohort. We demonstrated significant telomere elongation in lymphocytes exposed to HRE, associated with less toxicity than that induced by danazol treatment. This telomere elongation could be related to telomerase activation. The proportion of lymphocytes with short telomeres decreased significantly after exposure to either HRE or danazol in samples from all donors.
2. Materials and Methods
2.1. Preparation of the Hydroethanolic Root Extract of Astragalus mongholicus Bunge
Roots of A. mongholicus Bunge were collected in China in October 2015 and identified by Gilles Thébaud from the UniVegE service of the University of Clermont-Ferrand (France) in which a voucher specimen was deposited (CLF110821). CLF is registered in the Index Herbariorum of the New York Botanical Garden.
The liquid HRE of A. mongholicus Bunge evaluated in this study was produced by PiLeJe Industrie (France) according to the patented process WO2001056584A1. The batch used in this study (no. C-16K404) contained 30.7% of dry material containing 0.05% formononetin and 0.16% of astragaloside IV. The drug extract ratio (DER) of HRE, expressed as the ratio of the dry weight of the original fresh plant material to that of the resulting extract, was 3:1. After addition of glycerol, the A. mongholicus Bunge HRE corresponds to a formononetin-standardized extract of A. mongholicus Bunge (EPS Astragale, PiLeJe Laboratoire, France).
2.2. High-Performance Thin-Layer Chromatography (HPTLC) Analysis of A. mongholicus Bunge HRE
Standards were diluted in methanol at a concentration of 0.1 mg/mL for formononetin (Extrasynthèse, Genay, France) and 0.51 mg/mL for astragaloside IV (European Directorate for the Quality of Medicines & HealthCare, Strasbourg, France). A. mongholicus Bunge HRE without glycerol (4 mL) was diluted in 16 mL of a mixture of ethanol and water (50/50:v/v). The resultant solution was shaken and centrifuged for 3 min at 4400 rpm. The supernatant solution was transferred into individual vials and then subjected to HPTLC analysis.
HPTLC analysis was performed on 100.0 × 100.0 mm silica gel 60 F 254 HPTLC glass plates (Merck, Germany). Standard solutions and samples were applied to the plates in bands 8.0 mm wide using a CAMAG Automatic TLC sampler (ATS 4). The plates were developed in a CAMAG Automatic developing chamber (ADC2), derivatization being accomplished using a TLC plate heater and a CAMAG Chromatogram Immersion Device. The chromatograms were recorded by a CAMAG Visualizer with WinCATS software. The specific chromatographic conditions used for the three types of compounds analyzed are presented in
Supplementary Table S1.
2.3. Liquid Chromatography/Mass Spectrometry (LC/MS) Analysis of A. mongholicus Bunge HRE
Ultra-high performance liquid chromatography (UHPLC) analyses were performed on an Ultimate 3000 RSLC UHPLC system (Thermo Fisher Scientific Inc., Waltham, MA, USA) coupled with a binary pump (U3000 HPG-3400RS) and a diode array detector. Compounds were separated on an Uptisphere Strategy C18 column (25 × 4.6 mm, 5 μm; Interchim, Montluçon, France) and maintained at 40 °C. The flow rate was 0.8 mL/min, and the injection volume was 5 µL. Mobile phases were phase A, 0.1% (v/v) formic acid in water and phase B, 0.1% (v/v) formic acid in acetonitrile with the linear gradient: 0–25 min, 100%–0% of phase A. The UHPLC system was connected to a Q-Exactive Orbitrap (Thermo Fisher Scientific Inc.) mass spectrometer operated in negative and positive electrospray ionization modes. Source operating conditions were: 3 kV spray voltage for the negative mode and 3.5 kV spray voltage for the positive mode; 320 °C heated capillary temperature; 475 °C auxiliary gas temperature; sheath, sweep and auxiliary gas (nitrogen) flow rate 60, 18 and 4 arbitrary units, respectively, and collision cell voltage between 20 and 50 eV. Full-scan data were obtained at a resolution of 35,000, whereas tandem mass spectrometry (MS2) data were obtained at a resolution of 17,500. Data were processed using Xcalibur software (Thermo Fisher Scientific Inc.).
The components of the HRE were characterized according to their retention times, mass spectral data and comparison with authentic standards, when available, or otherwise with published data.
2.4. In Vitro Exposure of Cell Lines And Cytotoxicity Approach
Lymphoblastoid cells from two human cell lines (BJAB and DG-75) were treated with increasing doses of HRE (0.01, 0.1, 1 and 10 µg/mL) dissolved in ethanol 30% for 72 h at 37°C. For the controls, the same procedure was followed with ethanol alone (negative control) and with danazol (Sigma, Saint Quentin Fallavier, France; dissolved in DMSO to concentrations of 0.01, 0.1, 1 and 10 µg/mL; positive control). Survival was assessed using trypan blue, cell proliferation being evaluated on the basis of the mitotic index after cell arrest. The number of cells in metaphase and interphase were scored. The mitotic index was the ratio between the number of cells in metaphase to the total number of scored cells.
2.5. Peripheral Blood Lymphocyte In Vitro Exposure and Culture Conditions
Peripheral blood lymphocytes from 18 healthy donors (15 men and 3 women) with a mean age of 63.5 years (range 32–86 years) were used in this study. A large cohort of 150 healthy donors was used as a control. The use of samples from healthy donors has been approved by the Ethic Committee of Gustave Roussy Cancer Campus University Paris Saclay (ethical approval code: Comités de protection des personnes CPP 97/06, and Ile-de-France/ Nephrovir/ 2010). Informed consent was obtained from all donors included in this study.
Blood lymphocytes were exposed to 1 µg/mL HRE or danazol and cultured in RPMI 1640 medium (Gibco-BRL, Grand Island, NY, USA) supplemented with Glutamax, 10% fetal bovine serum (Eurobio, Courtaboeuf, France) and antibiotics (penicillin and streptomycin; Gibco-BRL) for 72 h (3 days) at 37 °C. The effects of A. mongholicus Bunge HRE and danazol on lymphocyte proliferation and telomere length were assessed.
2.6. Telomere Quantification
Telomeres were quantified in interphase cells using the quantitative fluorescence in situ hybridization (Q-FISH) technique with a Cy-3-labelled PNA probe specific for TTAGGG (Eurogenetec, Liège, Belgium), permitting investigation of intercellular variation in a large number of scored cells. The detailed procedure was described previously [
11,
12]. Quantitative image acquisition and analysis were performed using Metacyte software (Metasystem, version 3.9.1; Altlussheim, Germany). The mean fluorescence intensity (FI) of telomeres was automatically quantified in 10,000 nuclei on each slide. Settings for exposure and gain remained constant between captures. The experiments were performed in triplicate. Internal (cell line) and external (fluorescence beads) controls of the fluorescence intensity were used in each experiment. Telomere length, measured as mean FI, was also translated into the mean telomere length in kilobases (kb) using a standard curve performed in cancer patients, as well as in human cell lines using the telomeric restriction fragment (TRF) [
11] (
Supplementary Figure S1). Mean telomere length was expressed in kb.
2.7. Telomerase Expression Using Immunofluorescence
To quantify telomerase expression, peripheral blood lymphocytes of 6 donors were isolated in Ficoll medium (Ficoll, Biochrom AG, Berlin, Germany) and then cultured in RPMI medium (Gibco-BRL) supplemented with 10% fetal bovine serum (Eurobio, Courtaboeuf, France) and antibiotics (penicillin and streptomycin; Gibco-BRL) at 37 °C for 72 h. The detailed procedure was published previously [
33].
2.8. Statistical Analysis
All data were analyzed using R software version 3.5.3 and libraries. Mean comparisons were computed using the two-sample Wilcoxon test. The following convention for symbols indicating statistical significance were used: ns for
p > 0.05, * for
p ≤ 0.05, ** for
p ≤ 0.01, *** for
p ≤ 0.001 and **** for
p ≤ 0.0001. The regression curve presented was computed on the mean telomere length previously determined in a cohort of 150 healthy donors [
34] using a linear regression model (lm).